Case study: HeartFlow’s journey from NICE guidance to MedTech Funding Mandate

At NICE, we intend to be at the forefront of the drive to bring new and better treatments to patients and service users quickly. As part of this work, we’re placing significant focus on digital health over the next 5 years. In this case study, we speak to HeartFlow’s Gina McDonald Main and Campbell Rogers about their product’s journey through our Medical Technologies Evaluation Programme.
“NICE is respected globally. Working with them has been such a positive experience.”
Gina McDonald Main, vice president of government affairs at HeartFlow.
“NICE is known for its rigorous, unbiased and objective approach. Overall, I thought it was an amazing process.”
Campbell Rogers MD, executive vice president and chief medical officer at HeartFlow.

About the technology

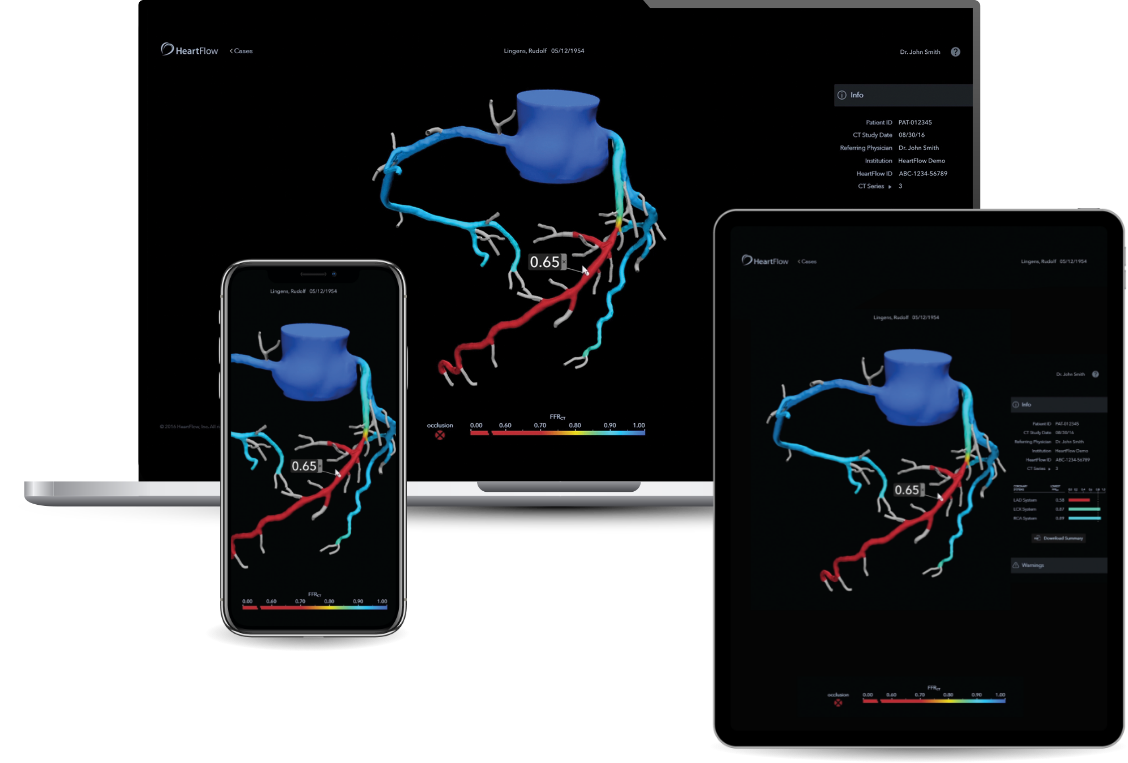
HeartFlow is an artificial intelligence-enabled service that analyses images from a cardiac CT scan.
This non-invasive digital diagnostic tool helps health professionals in determining the extent of a coronary artery’s narrowing and the impact that the narrowing has on blood flow to the heart.
By helping physicians identify which patients do and do not need intervention, HeartFlow can reduce unnecessary invasive testing, improve patient experience and reduce both waiting times and healthcare costs.
NICE Medical Technologies Evaluation Programme

Our Medical Technologies Evaluation Programme produces guidance on technologies that offer benefits to patients and the health and social care system over current practice. In 2014, NICE approached the developers of HeartFlow with a view to taking it through guidance production. Importantly, the technology met the programme’s eligibility criteria and the benefits were plausible, clearly described, easily understood and supported by evidence.
“We had received a CE mark for our product in July 2011,” begins Campbell. “We had published extensive clinical data in peer reviewed journals and by virtue of that, we were able to be totally transparent. I think that stood us in good stead when it came to NICE. Going into the process, we had confidence in both our technology and our clinical data.”
A rigorous approach

Our medical technologies advisory committee gave the go ahead for guidance production in 2015. HeartFlow then had to complete their evidence submission. “NICE is known for its rigorous approach and preparing our evidence was a lot of work,” Campbell acknowledges. The HeartFlow team carried out 2 major literature reviews and developed a large-scale economic model. NICE then used King’s Technology Evaluation Centre, an independent external assessment centre at Kings College London, to review and critique the evidence provided.
“It was very thorough,” agrees Gina. “NICE dots every ‘i’ and crosses every ‘t’. It’s an incredible process, all done in such an organised and professional way.”

Medical technologies advisory committee

Here at NICE, independent committees develop and update our guidance. Each committee is made up of a diverse range of members including people who use health and social care services, carers and health and social care practitioners. It is their vital work that helps shape our recommendations.
Campbell was struck by the diversity of voices that made up our medical technologies advisory committee. “All the committee members were very engaged,” he reveals. “I thought the comments they made were fair and objective. Their broad backgrounds meant they often gave fresh perspectives.”
Gina was impressed that lay members were included on the committee. “I thought it was fantastic that there were patients in the committee room,” she enthuses. “When the benefits of HeartFlow were explained, you could see their eyes light up. They recognised how convenient it could be. HeartFlow has the potential to make a huge difference to patients and that’s the most important thing.”

A fair and balanced assessment

The successful adoption of HeartFlow is dependent upon the availability of adequate CT scanners and the number of professionals trained in CT coronary angiography. Campbell and Gina were pleased that potential adoption challenges did not unduly influence the appraisal.
“Large-scale healthcare systems cannot just turn a switch and have everybody suddenly work in different ways,” Campbell states.
“This inescapable truth could easily have distracted the committee. But the committee chair did not let that happen. Under his direction, the committee remained focused on conducting a fair and balanced assessment of HeartFlow’s benefits based on the clinical and economic evidence.”
To help alleviate these implementation challenges, our health technology adoption team worked with the NHS and other system partners to produce a HeartFlow adoption support resource.
This valuable tool summarises the learning and experiences reported by NHS providers that have adopted HeartFlow. Funding and support for HeartFlow has also been made available through the NHS Accelerated Access Collaborative.
A flexible process

Throughout the process, Gina and Campbell found NICE helpful and supportive. A good example of this relates to a HeartFlow trial that was completed during the evaluation process. The results were definitive but were yet to be published or peer reviewed.
“We were obviously keen to share this powerful data confidentially with the committee,” Campbell says. “NICE agreed to take the trial results into consideration alongside the extensive published evidence we’d already submitted. I was impressed with their open-minded approach.”
A positive recommendation

NICE published positive guidance on HeartFlow for estimating fractional flow reserve from coronary CT angiography in February 2017.
“Globally, NICE and the UK were the first to embrace HeartFlow and the new care pathway,” Gina declares. “They were ahead of the curve.”
Campbell agrees. “The rest of the world has now come around to what NICE introduced back in 2016 and 2017 with its chest pain clinical guideline and HeartFlow medical technologies guidance. Years later, in October 2021, The American College of Cardiology and the American Heart Association released new guidelines which are very similar to NICE’s. They place coronary CT angiography front and centre. NICE and the NHS were way ahead on this.”

MedTech Funding Mandate

HeartFlow has also benefited from being included in the NHS Accelerated Access Collaborative MedTech Funding Mandate. The mandate is an NHS Long Term Plan commitment to give patients access to selected NICE-approved cost-saving devices, diagnostics and digital products more quickly. HeartFlow is 1 of the 4 technologies supported in 2021/22.
“We are proud, honoured and delighted to be part of the MedTech Funding Mandate,” says Gina. “Hospitals that were facing challenges in adopting HeartFlow are now coming to us saying: ‘This is mandated. We need to provide this for our patients.’”
Words of wisdom

For other digital health developers who are exploring how best to introduce their products to the NHS, Campbell concludes by offering some sound advice.
“Get the data,” he asserts. “Do not seek approvals prematurely until you can prove your point. If you have the data to hand, NICE will listen.
"They are an open ear for innovative solutions that offer real value to patients and the NHS.”
